Business & Strategy Consulting
|
- Corporate strategy consulting
- Therapy area strategy consulting
- Product development plan consulting
|
Genomics / Translational Medicine
|
- Precision Medicine
- Translational medicine
- Genotype
- Outcomes / response predictors
- Computational biology
|
Study Optimization
|
- Population targeting
- Study design
- Endpoint selection
- Unmet need
- Time – cost – quality modeling
- Alternative models
|
Patient & Site Recruitment
and Feasibility
|
- Eligibility criteria modeling
- Feasibility assessment
- Enrollment and timeline assessment
- Probability of success assessment
|
Patient Safety / Pharmacovigilance
|
- Pre- and Post- market authorization Case processing of clinician reported adverse event data
- Regulatory reporting
- Safety signal detection
- Evolving to address better sampling of data, workforce efficiency, more robust analysis
|
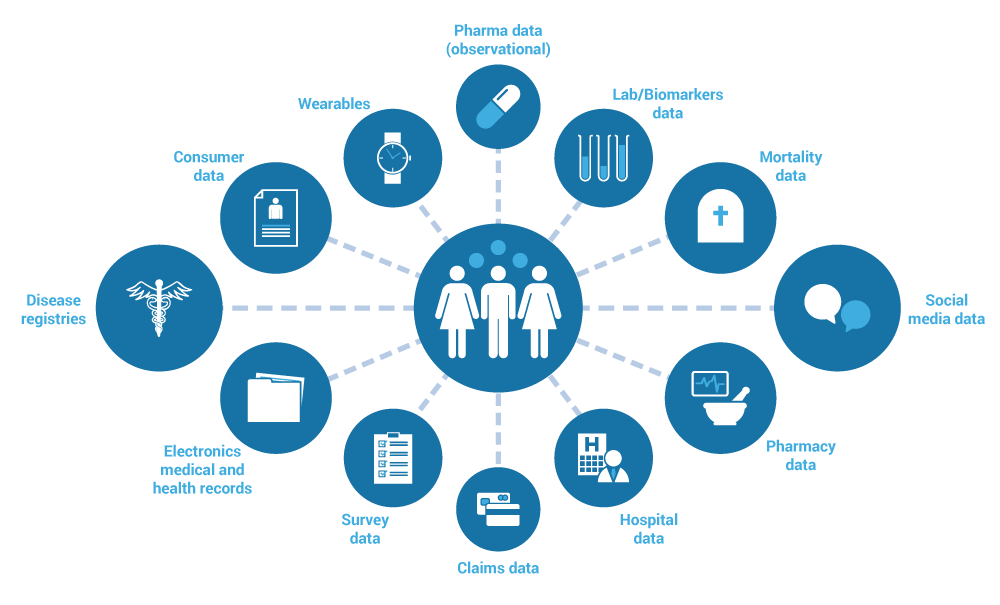
Real-World Evidence Generation
|
- Standard of care based research
- Outcomes based studies
- Label expansion studies
- Hybrid data models
- Virtual studies
- EMR based cohort models
- Direct with Patient studies
|
Market Access Planning
|
- Health economic modeling
- Landscape assessment
- Pricing and market access consulting
- Product value
- HTA panels, submissions
- Product development strategy
|